(Stephanie Watson/ Web MD) — The U.S. FDA has approved the first in a new class of migraine drugs that aim to fight painful migraine headaches before they start.
Erenumab (Aimovig) is the first of four new migraine drugs in the pipeline that target calcitonin gene-related peptide (CGRP), a molecule that’s produced in nerve cells of the brain and spinal cord.
“Aimovig provides patients with a novel option for reducing the number of days with migraine,” said Eric Bastings, MD, deputy director of the Division of Neurology Products in the FDA’s Center for Drug Evaluation and Research. “We need new treatments for this painful and often debilitating condition.”
The list price will be $575 a month, or $6,900 annually.
About 39 million Americans live with the throbbing pain and nausea of migraine. More than 4 million of them have at least 15 migraine days each month. Even though many drugs prevent and treat these headaches, migraine symptoms disable 1 in 5 people who get them.
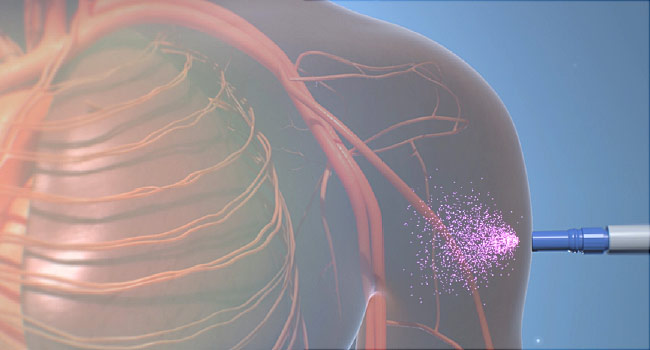
Aimovig is a monoclonal antibody given as a shot for people who have four or more migraine days each month. The FDA evalauted results of three patient studies in making its approval. In those studies, patients on average had one to 2 ½ fewer monthly migraine days either over six months or three months. (…)